INTRODUCTION
Shoulder injury related to vaccine administration (SIRVA), is an avoidable sequelae of vaccine placement. Although the far majority of shoulder discomfort from shoulder vaccinations are temporary and self-limited, there remains a risk for potential injury or irritation of shoulder structures from errant injections and / or tissue response to the delivered vaccine. Understanding the anatomy of the shoulder and the symptoms related to SIRVA are important to avoid unwanted complications from vaccine injection placement. In litigation, a vaccine shoulder injury (SIRVA) expert witness may be necessary to opine as to the cause of the injury.
BACKGROUND
The United States Department of Health and Human Services has an annual goal of vaccinating 70% of the population annually for influenza – aka, the “flu shot”.1 Generally, nearly 50% of the population receives the flu shot annually, which represents over 150 million people per year.1 Now with the COVID-19 Pandemic, the Center for Disease Control similarly is aiming for a vaccination goal of 70% of the population.2 Moreover, depending on which vaccination is given to an individual, the COVID-19 vaccination may also require up to two injections approximately a month apart. It is also anticipated that the recommendation will be to receive an annual COVID-19 booster injection as well. These two vaccinations, Influenza and COVID-19, represent the most common vaccinations but do not account for all vaccinations given annually in the United States. As more of the population receives vaccinations, it is estimated that Americans will receive nearly 500 million vaccinations annually.3 Subsequently, there may be more instances of vaccine shoulder injuries and a (SIRVA) expert witness may be needed to opine on the cause of injury.
ANATOMY
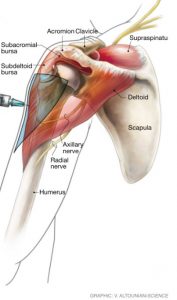
Most vaccinations, including Influenza and COVID-19, require intra-muscular placement of the vaccine into the deltoid muscle of the shoulder. The deltoid muscle is the large outer muscular covering of the shoulder joint. The joint itself consists of the gleno-humeral “ball and socket” joint, the surrounding shoulder capsule, and the overlying rotator cuff and surrounding subacromial bursa (see Figure). Surrounding the shoulder joint but deep to the deltoid muscle are also two important nerves – the Axillary and Radial nerves. An errant injection can potentially injury any of these structures. An injection placed too deep into the shoulder capsule can cause shoulder joint inflammation (synovitis) or an infection (septic arthritis). An injection placed into the rotator cuff can cause a rotator cuff injury (tendonitis or tear of the rotator cuff). An injection placed into the subacromial space can cause a shoulder bursitis and / or frozen shoulder (adhesive capsulitis). An injection placed into the axillary or radial nerve can cause nerve irritation, tingling, numbness, and weakness of the hand and arm. The placement of an injection may or may not comport with the applicable standard of care. A vaccine shoulder injury (SIRVA) expert witness may be needed to opine as to the applicable standard of care for injection placement and whether such standard was met.
SYMPTOMS
The majority of shoulder pain following a vaccination is temporary and will resolve within 1 day to 1 week of the injection with time, rest, and over-the-counter medications. However, in the case of SIRVA, pain will persist and even worsen with time. Common complaints will include pain at rest, increased pain with active shoulder motion, loss of shoulder range of motion and strength, infection, and radiating pain or tingling distally into the extremity.
TREATMENT
Keys to prevention of SIRVA include a general understanding of the anatomy of the shoulder, palpating the shoulder prior to injection placement to assess the anatomy for optimal vaccine injection placement, and adjusting needle length and depth relative to the size of the patient.
When a patient does present with SIRVA complaints, the first step in treatment is physical examination to assess what is the most likely injured structure. Radiographs can also be taken, but advanced imaging with an MRI is preferred to better assess the various structures susceptible to injury from the vaccination needle. Treatment is rendered based on the combination of history, physical examination, and MRI findings. The typical and preferred treatment initially is non-operative. However, in certain cases operative intervention may be necessary.
All these issues may be reviewed by a vaccine shoulder injury (SIRVA) expert witness to determine the nature and extent of the injury and whether the administration of the vaccine met the applicable standard of care.
About the Author
Thought leading orthopedic surgeon. Active clinical practice at the Rothman Institute in Philadelphia focusing on hand, wrist, elbow, shoulder, and nerve injuries. Full Professor at Thomas Jefferson University. Harvard trained. Recipient of numerous honors and awards. Author of over 150 articles and 15 book chapters. Editor/reviewer for multiple journals. Frequent presenter at national meetings. Past President of the Pennsylvania Orthopaedic Society. Over 10 years expert witness experience, including regular deposition and trial testimonies.
REFERENCES: