Hypoxic-ischemic encephalopathy (HIE)
DEFINITION
Neonatal encephalopathy is a clinical syndrome characterized by “a subnormal level of consciousness or seizures, and often accompanied by difficulty with initiating and maintaining respiration and depression of tone and reflexes.” (Swaiman) When it is caused by deprivation of oxygen to the brain, the diagnosis is hypoxic-ischemic encephalopathy (HIE). Although often said together as hypoxic-ischemic, it is important to understand these two causes of oxygen deprivation. Hypoxia is defined as the deficiency in amount of oxygen that is being delivered due to reduced oxygen in the blood, whereas ischemia is an inadequate blood supply.
TERMS DEFINED
Acidemia – low blood pH
Sentinel event – an unanticipated event resulting in death or serious injury
Peripartum – around the time of birth
Intrapartum – the onset of labor through the delivery of the placenta.
CAUSATION OF HYPOXIC-ISCHEMIC ENCEPHALOPATHY (HIE)
In Neurology of the Newborn 6th edition by Volpe the “neurologic syndrome that accompanies serious peripartum hypoxic-ischemic cerebral injury is the prototype for neonatal HIE” (Volpe).
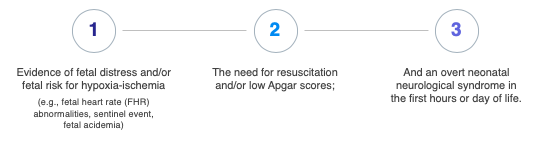
When looking at the causation and timing of a neonatal hypoxic-ischemic brain injury, three features are most important. First is evidence of fetal distress (fetal heart rate abnormalities, sentinel event, fetal acidemia). The second feature is the need for resuscitation (the supportive measures used to revive a neonate after being born depressed) or low APGAR scores. The third is an overt neonatal neurologic syndrome, an encephalopathy, in the first hours and days of life.
FETAL DISTRESS
Fetal distress can be evidenced by fetal heart rate abnormalities, a sentinel event, or fetal acidosis. Fetal heart rate abnormalities, can be seen on the fetal heart tracing prior to the delivery and in general are reviewed and opined upon by obstetrical clinicians. In a minority of patients with a neonatal hypoxic-ischemic encephalopathy (HIE), a sentinel event is noted, including uterine rupture, cord rupture, cord prolapse, and placental abruption. These all can lead to sudden decreased oxygen or blood delivery to the fetal brain. Fetal acidosis is seen in the cord blood taken at the time of delivery or in subsequent blood gases following the delivery. (ACOG) Fetal acidosis is caused by tissue not receiving adequate oxygen and having to utilize glucose in an anaerobic environment (lacking oxygen) and producing lactic acid instead of using the normal process (aerobic) where oxygen is present and glucose is broken down to carbon dioxide and water.
Fetal acidemia, in a neonate with a possible brain injury secondary to loss of oxygen, usually shows signs of an abnormally low pH or high base deficit. In order to understand metabolic acidosis as it relates to fetal distress, an understanding of where the acid is produced versus where it is measured is needed. The acid is produced in the cells within the fetal tissue, generally in muscle. In order for it to be tested, the acid needs to move from the tissue into the fetal blood and onward into the umbilical cord (for cord blood testing). There are times when a fetus has sustained such poor circulation that the acid is not initially able to be documented on a cord blood gas. The acid produced in the tissue may not cross into the bloodstream until after the child is born and resuscitated (adequate circulation restored). This is seen when there is a relatively normal cord blood gas and there was a vigorous resuscitation and a subsequent blood gas is shown to be worse. (ACOG)
RESUSCITATION
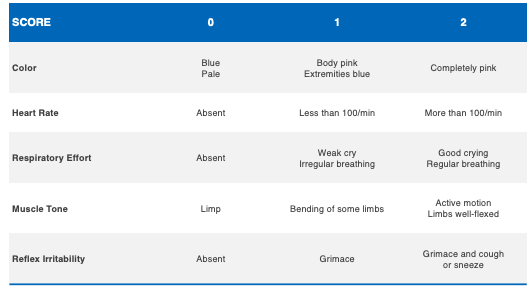
The need for resuscitation is often seen in neonates with low one minute, five minute, and 10 minute APGAR scores. APGAR scores range between zero and 10. An APGAR score has five categories, which are noted in the table below.
These include color, heart rate, respiratory effort, muscle tone and reflex irritability. When the child requires resuscitation following delivery, this can include positive pressure ventilation using a bag and mask to assist breathing, intubation using a tube and ventilator, chest compressions to assist in circulation, and epinephrine (adrenaline) to increase the heart rate and blood pressure.
ENCEPHALOPATHY
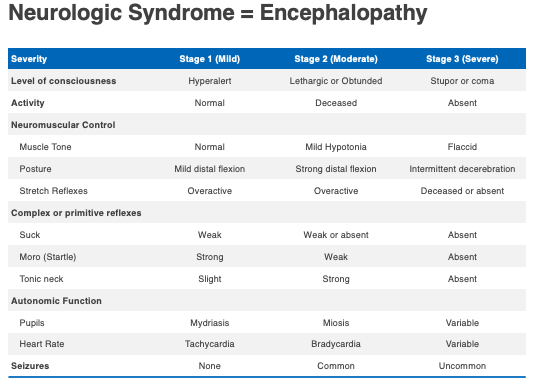
The third feature is the neurologic syndrome or encephalopathy. As noted below in the table below, it ranges from mild (stage one) to severe (stage three). The major features of encephalopathy include lethargy, abnormal tone, difficulty feeding, and if moderate or severe, can include seizures.
TREATMENT
The treatment for a child with neonatal hypoxic-ischemic encephalopathy (HIE) includes hydration, supporting blood pressure, and assisted breathing. Seizures, when present, can cause further brain injury and need to be diagnosed and treated. Lastly, hypothermia, which is actively reducing the temperature below normal (33.5 degrees centigrade for 72 hours), has been shown to reduce the future risk of injury in neonates that have sustained a hypoxic brain injury. Hypothermia is most effected when started within the first six hours of life in neonates with moderate to severe hypoxic-ischemic encephalopathy (HIE).
TIMING
In a child with a neonatal hypoxic-ischemic encephalopathy (HIE), timing of the event is an important feature to define. Volpe notes that 56% of all cases of newborn encephalopathy were related to hypoxic-ischemic injury that occurred during the intrapartum period. Less than 20% were due to antepartum events. 15% of cases of intrapartum hypoxic-ischemic injury are related to a sentinel event. (Volpe)
IMAGING OF THE BRAIN
MRI of the brain is useful when a child has a suspected perinatal hypoxic-ischemic encephalopathy (HIE). Most patients, following this type of injury, have one of two types of brain injury seen on MRI. These are profound hypoxic-ischemic injury (HII) and partial hypoxic-ischemic injury. Profound can involve the perirolandic cortex, white matter, hippocampi, midbrain, dorsal brainstem, thalamus and basal ganglia. Partial HII can involve cortex (symmetric or asymmetric) along with subcortical white matter. (Barkovitch) Not all cases of HIE result in an abnormal MRI. Cowan et al. as cited by Volpe note that 80% of neonates had MRI evidence of an acute peripartum lesion consistent with hypoxic-ischemic insult, while 16% had normal MRI scans. (Volpe)
OTHER POSSIBLE CAUSES THAT ARE NOT INTRAPARTUM HYPOXIC-ISCHEMIC ENCEPHALOPATHY (HIE)
When looking at a child with neonatal encephalopathy, it is important to look at other causes that may mimic an intrapartum hypoxic-ischemic encephalopathy (HIE), which include events happening prior to the start of labor (antepartum), events happening after the child is born (postpartum), underlying genetic/congenital conditions, metabolic disorders and infections, including sepsis. Antepartum causes can include trauma and infections, including toxoplasmosis, syphilis, rubella, cytomegalovirus (CMV) and herpes infections. Postpartum difficulties can include infections (meningitis, sepsis), as well as metabolic disorders like hypoglycemia and difficulties with resuscitation that lead to a hypoxic brain injury following the delivery. When looking for a possible genetic or congenital abnormality, dysmorphic features may be present including unusual facial features, other organ abnormalities, brain malformations, and skin lesions consistent with a syndrome.
Case Example #1
Born at 40 weeks gestation after a normal pregnancy. Fetal bradycardia was noted for 24 minutes prior to the delivery. The child was born by stat caesarian section, and the mother was found to have a placental abruption. Following the delivery, the child was noted to be floppy, not breathing, not moving and blue. APGAR scores at 1, 5 and 10 minutes, were 1, 2 and 2 respectively. Following delivery, the child required positive pressure ventilation with bag and mask, chest compressions, epinephrine and was intubated. The child’s arterial cord gas at the time of delivery showed a pH of 6.92 with a base deficit of 26. The child began having seizures at two hours of life. Following the delivery, the child had symptoms of a severe encephalopathy, including no gag reflex, no movement and the pupils were not reactive. The child died at seven days of life due to severe hypoxic ischemic brain injury. This case represented a severe hypoxic-ischemic encephalopathy.
Case Example #2
Born at 40 weeks gestation by cesarean section due to non-reassuring fetal heart rate. The labor was noted to be prolonged with greater than 24 hours of ruptured membranes. There was a maternal fever starting two hours prior to delivery, and the mother was diagnosed with chorioamnionitis. Following the delivery, the placenta showed signs of chorioamnionitis on the pathologic examination. APGARs were 3, 5 , and 7. Cord arterial gas was pH 7.21 base deficit of 6. Resuscitation following the delivery included positive pressure ventilation followed by intubation. The child showed low tone, poor respiratory effort, and was admitted to the neonatal intensive care unit. At one hour of life, the child had a lactate of seven, which was elevated and a pH of 7.07 with a base deficit of 13. The child was not able to feed for several days, had low tone, but was moving and pupils were reactive. No seizures were noted following the delivery. The child was cooled for 72 hours and subsequently had an MRI of the brain performed, which was normal. This case represented a moderate hypoxic ischemic encephalopathy.
Case Example #3
Child was born at 39 and 6/7 weeks by vaginal delivery without any reported fetal distress. Arterial cord pH was 7.23 with a base deficit of three. APGARs were 6, 2, 1, 2, 3 at 1, 5, 10, 15, 20 minutes, respectively. Initially the baby was vigorous, but had difficulty breathing and became apneic following delivery. Positive pressure ventilation, chest compressions, intubation at 15 minutes of life were performed. This case represented a child that likely had a hypoxic event following the delivery.
CONCLUSION
An understanding of the evidence of fetal distress, the need for resuscitation, an overt neonatal neurological syndrome, and the timing of the events will assist in helping determine hypoxic-ischemic encephalopathy (HIE) causation.
ABOUT THE AUTHOR
Brian Woodruff, MD is a pediatric neurologist with a specialty focus in Pediatric Neurology, Child Neurology, Hypoxic-ischemic encephalopathy (HIE), cerebral palsy and strokes. He received his M.D. from Michigan State University – College of Human Medicine and completed his Pediatric Residency at University of Wisconsin; Pediatric Neurology Fellowship, University of Michigan. He is a Diplomat, American Board of Psychiatry and Neurology with Special Qualification in Child Neurology; Board Certified Pediatric Neurologist; Pediatric Residency: University of Wisconsin; Pediatric Neurology Residency: University of Michigan. He can be reached at dr.woodruff@gmail.com or 734-347-6579.
- Swaiman, K. F. (2012).Swaiman’s Pediatric Neurology: Principles and Practice.
- Volpe, J. (2018). Volpe’s Neurology of the Newborn, 6th Edition
- Barkovitch, J (2017). Diagnostic Imaging: Pediatric Neuroradiology, 2nd Edition
- Neonatal Encephalopathy and Neurologic Outcome. 2014
- Jenster, et al., Maternal or neonatal infection: association with neonatal encephalopathy outcomes, Pediatr Res 2014 July; 76(1): 93–99.